Nutrient availability for the plants depends on how acid or basic the soil solution is. Fertilizers that either provide for the plants grown in basic soils the nutrients that would only be available in acidic soil or help to lower the soil pH, are known as acidic fertilizers. Influence of soil solution’s pH in nutrient availability There are many factors involved in the soil’s acidity. How alkaline or acidic the parent material is, if the soil bases were leached or removed during harvest, the amount of organic matter decomposed, the occurrence of acid rain, and of course, the use of acidic fertilizers all need to be considered when trying to reach the ideal soil pH for your crop. Tropical soils, for example, are usually more acid, granted that the high rainfall can leach most of the base cations, and there aren’t enough primary and secondary minerals to replace those bases.
It is important to know your soil’s pH because it is a determining factor for the development of some crops and it can dictate if nutrients are available to be absorbed by the plants, or not. This information is obtained through a soil test, that can be interpreted and help to define the best course of action for your crop. With i-Plant Nutrition software, all you need to do is insert the soil test values and we will take care of the rest!
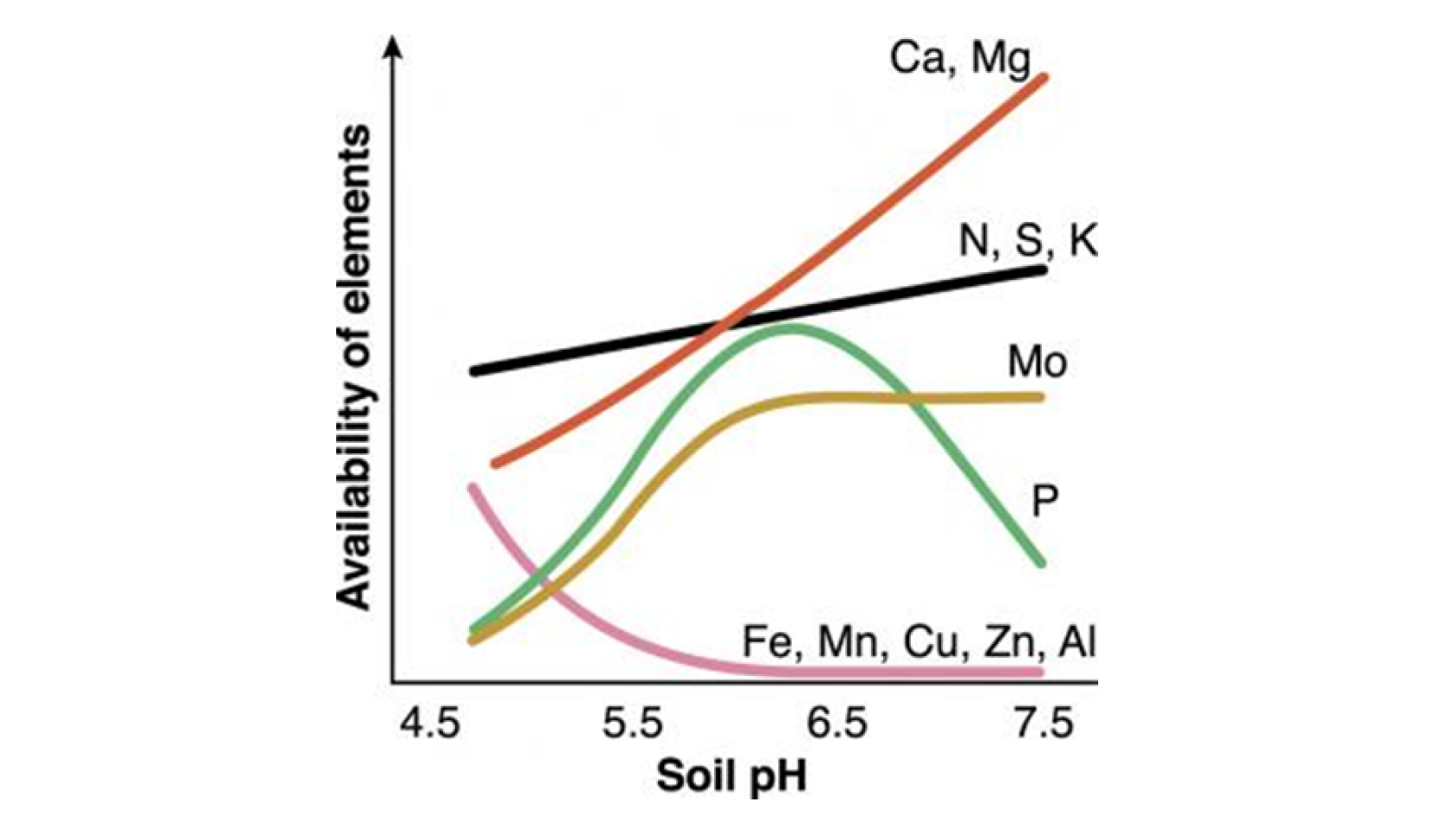
Figure source: Government of Western Australia- Department of Primary Industries and Regional Development
Macronutrients (N, P, K, Ca, Mg and S) and anionic micronutrients (Cl and Mo), in other words, the majority of the plant’s essential nutrients, are more available in non-acidic soils, near a neutral pH, between 6 and 6,5. Consequently, this is the default range of soil pH for most crops, and whenever the soil is too acidic, farmers turn to limestone to increase the pH, attempting to get closer to neutrality and boost their plants’ nutrition.
Although this may be true, there are a few nutrients that benefit from soil acidity. Zinc, Iron, Copper, Manganese and Nickel, known as cationic micronutrients given their positive charges, need lower soil pH to be readily available for the plants. The more basic the soil, the more they precipitate into oxides and become inapt to be absorbed.
Understand the role of each cationic micronutrient:
- Copper: responsible for the lignification of the cell wall and reduction of oxygen in photosynthesis.
- Iron: essential for the energy metabolism
- Manganese: acts on the chlorophyll synthesis
- Zinc: related to plant growth and the development of floral structures
- Nickel: acts on the nitrogen metabolism
For a fast pH reduction, it is ideal to look for nitrogen fertilizers, for example, anhydrous ammonia, ammonium nitrate, nitrocalcium and urea. The most common phosphorus fertilizers for this purpose are superphosphate and triple phosphate. Sulfur can be used as ammonium sulfur or ammonium sulfonitrate.
Before applying any of those fertilizers it is very important to read the instructions carefully, seeing that, some of them, ammonium sulfur, for instance, are quite strong and can easily burn the plant.
If the goal is to lower the soil’s pH for the long term, the use of elemental sulfur might be the best choice. The bacteria in the soil oxidize sulfur molecules inducing them to release hydrogen ions, acidifying the soil. The application should happen at least a year before planting and the sulfur needs to be incorporated on the ground owing to the fact that it takes months to act.
Instead of acidifying the soil to release nutrients that were previously unavailable, acidic fertilizers can also deliver those nutrients (usually in chelated form) directly to the deficient plant through the foliar application, causing only a minor drop in the soil’s pH. Acid-loving plants Most of the plants that thrive under acidic circumstances are ornamental, such as azaleas, camellias, gardenias, crepe myrtle, bleeding heart and calla lilies. Then again, blueberries and pine trees are also examples of acid-loving plants that might need acidic fertilizers to improve their nutrition.
 The best way to know if your acid-loving plant is struggling to absorb essential nutrients given the soil’s
alkalinity is to pay attention to the leaves. Lack of iron will show on the young leaves as chlorosis with green
veins. If the young leaves are shrivelled and twisted, it can be a sign of copper deficiency.
The best way to know if your acid-loving plant is struggling to absorb essential nutrients given the soil’s
alkalinity is to pay attention to the leaves. Lack of iron will show on the young leaves as chlorosis with green
veins. If the young leaves are shrivelled and twisted, it can be a sign of copper deficiency.
However, there is no need to wait for your plant to show visual signs of deficiency. With the i-Plant Nutrition, you can get an instant evaluation of the nutrient status of the crop by simply uploading a leaf test. With the results, we can create a customized fertilization plan based on how the plants are taking up nutrients.
Check our software here (Link to software tour)